how many valence electrons in phosphorus|How many valence electrons does phosphorus have? : Manila The elements that have 5, 6, or seven electrons in the last shell receive the electrons in the last shell during bond formation. . Tingnan ang higit pa Lottery.co.uk; Lottery Results; Here you can find the results for the most popular lotteries, including all National Lottery games plus independent society lotteries. This page is updated straight after each draw takes place, so you can be the first to find out if you are a winner! Check your tickets, then select a result to view more prize .
PH0 · Valence electrons (video)
PH1 · Valence Electrons Chart for All Elements
PH2 · Valence Electrons
PH3 · How to Find the Valence Electrons for Phosphorus (P)?
PH4 · How many valence electrons does phosphorus have?
PH5 · How many valence electrons does phosphorus have?
PH6 · How many valence electrons are in an atom of phosphorus?
PH7 · How Many Valence Electrons Does Phosphorus (P) Have? [Valency of
PH8 · How Many Valence Electrons Does Phosphorus (P) Have?
PH9 · How Many Valence Electrons Does Phosphorus (P)
PH10 · 4.7: Arrangements of Electrons
PH11 · 2.9: Valence Electrons
PH12 · 18.9: The Chemistry of Phosphorus
Derby Lotto Prediction for Today . The Derby Lotto is a lottery game that is completely random, and there is a slim chance to predict the winning numbers. However, there are some statistics about the Derby Lotto that may be helpful in making your own predictions. The Derby Lotto has 6 winning numbers, which are drawn from a pool of 1-49.
how many valence electrons in phosphorus*******Learn how to find the valence electrons of phosphorus (P) by following three steps: determining the total number of electrons, arranging them in shells, and identifying the valence shell. Also, learn about the valency, ionization, and compound formation of phosphorus. Tingnan ang higit paThe second element in group-15 is phosphorus. The valence electron is the total number of electrons in the last orbit. The total . Tingnan ang higit paThe valence electrons have to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electron . Tingnan ang higit paThe elements that have 5, 6, or seven electrons in the last shell receive the electrons in the last shell during bond formation. . Tingnan ang higit pa
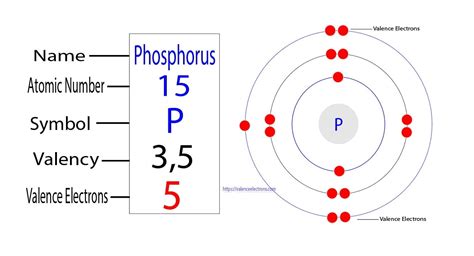
The ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). The number of unpaired electrons in the last orbit of an element is the valency of that element. Ground state electron configuration . Tingnan ang higit pa According to the periodic table above, phosphorus belongs to Group 5A. Therefore, Its valence electrons should be 5. Let's check using the electron configuration: 1s^2 2s^2 2p^6 3s^2 3p^3 = 15 electrons = . Mar 23, 2023
How many valence electrons does phosphorus have? In fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. Neon, with its configuration ending in 2s22p6 . Learn how to find the valence electrons and valency of phosphorus (P), a nonmetal with atomic number 15 and electron configuration [Ne]3s²3p³. Phosphorus has five valence electrons and a .
Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as .The valence electrons are the electrons that determine the most typical bonding patterns for an element. These electrons are found in the s and p orbitals of the highest energy .
As a member of the Nitrogen Family, Group 15 on the Periodic Table, phosphorus has 5 valence shell electrons available for bonding. Its valence shell configuration is 3s 2 3p 3. Phosphorus forms mostly covalent .A neutral phosphorus atom has 15 electrons. Two electrons can go into the 1 s subshell, 2 can go into the 2 s subshell, and 6 can go into the 2 p subshell. That leaves 5 electrons. Of those 5 electrons, 2 can go into the .Total valence electrons given by phosphorous atom = 5; There are four oxygen atoms in PO 4 3-ion, Therefore. Total valence electrons given by oxygen atoms = 6 *4 = 24; There are -3 charge on PO 4 3-ion. . When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most .
Carbon has four valence electrons and here a valence of four. Each hydrogen atom has one valence electron and is univalent. In chemistry and physics, . For example, the electronic configuration of phosphorus (P) is 1s 2 2s 2 2p 6 3s 2 3p 3 so that there are 5 valence electrons .Step1: Calculate the total number of valence electrons available. Let’s use PO 4 3-as our example. We need to know how many electrons are available to make the bonds for Phosphate Ion. Phosphorus is in group VA so it has 5 valence electrons and Oxygen is in group VIA so each oxygen has 6 valence electrons. Total valence electrons = 5 + .
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) . Valence electrons are also the determining factor in some physical properties of the elements. Elements in any one group (or column) have the same number of valence .From the electron configuration of neutral phosphorus atoms in Example \(\PageIndex{1}\), how many valence electrons and how many core electrons does a neutral phosphorus atom have? Solution The highest-numbered shell is the third shell, which has 2 electrons in the 3 s subshell and 3 electrons in the 3 p subshell.
Phosphorus Valence Electrons Dot Diagram. The atomic number of a Phosphorous atom is 15. The 1s shell contains 2 electrons, the 2s shell contains 2 electrons and the 2p shell contains 6 electrons. So the outermost shell contains 5 valence electrons. So these 5 electrons can be distributed in the following manner: 2 can go .

The valence electrons are the electrons that determine the most typical bonding patterns for an element. These electrons are found in the s and p orbitals of the highest energy level for the element. Sodium 1s22s22p63s1. Sodium has 1 valence electron from the 3s orbital. Phosphorus 1s22s22p63s23p3. Phosphorus has 5 valence electrons 2 from the .
sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group . How many electrons does a phosphorus atom have? Electrons are the permanent core particles of an atom. It resides in a specific orbit of the atom and revolves around the nucleus. . The last shell of phosphorus has three unpaired electrons, so the valency of phosphorus is 3. The last electron of phosphorus enters the p-orbital. .
In the phosphate ion we have a central Phosphorus atom, with five valence electrons. This is bonded to four oxygen atoms, which have six valence electrons. Five P electrons plus 4 times 6 O electrons gives . This table of element valences includes the maximum valence and most common valence values in chemistry. . (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. Remember an element's electron cloud will become more stable by filling, emptying, or half-filling the .
During the formation of a bond, the last shell of phosphorus receives three electrons and turns into a phosphide ion (P 3- ). That is, phosphorus is an anion element. P + 3e – → P 3-. The electron .how many valence electrons in phosphorus How many valence electrons does phosphorus have? The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) . Valence electrons are also the determining factor in some physical properties of the elements. Elements in any one group (or column) have the same number of valence electrons; the alkali .how many valence electrons in phosphorus To determine the valence electrons of PCl 3, it is first necessary to know the valence electrons of the chlorine and phosphorus atoms. To determine the valence electrons of phosphorus trichloride we have to follow two steps. It is shown below: Step 1: Determine the valence electrons of phosphorus and chlorine atoms. The atomic .
The valence electrons in phosphorus are 5. Since 4 phosphorus atoms are present the total phosphorus valence electrons will be 5×4=20 electrons. What happens is the phosphorus atoms are placed in tetrahedral manner and then they are linked such that the valency of each of the phosphorus atom is satisfied. Phosphorus .
To determine the valence electrons of phosphorus pentachloride we have to follow two steps. It is shown below: Step 1: Determine the valence electrons of phosphorus and chlorine atoms. The atomic number of phosphorus is 15. So its total number of electrons is fifteen.
As a member of the Nitrogen Family, Group 15 on the Periodic Table, phosphorus has 5 valence shell electrons available for bonding. Its valence shell configuration is 3s 2 3p 3. Phosphorus forms mostly covalent bonds. Any phosphorus rock can be used for the production of elemental phosphorus. Crushed phosphate rocks and sand (\(\ce{SiO2 .
Categories - PinayFlix
how many valence electrons in phosphorus|How many valence electrons does phosphorus have?